“Oh my dear, I say, I have taken you be surprise,” Colon said, watching as a myriad of
emotions flit across the face of the young woman he held in his brawny
arms. “You’re the most beautiful woman I
have ever seen in my life, but I want you to know that it is not only your
beauty that has drawn me to you.” He
paused, reflecting, and then added, “ Lord, I sound such a fool. . . . to think
that you, such a ravishing creature,
might consider a plain, ordinary man
like me, and become Lady Falcon’s Nest.”
Catherine looked up into Colon’s deep blue
eyes. Her eyelids fluttered and she
spoke in a throaty, hesitant voice, “This is so sudden.” She shrugged her
alabaster shoulders out of Colon’s embrace and turned away, paused, and then
turned back and stared into those eyes once again, the eyes of Colon, the young
Duke of Falcon’s Nest. Her heart raced
within her bosom. No one had ever spoken
to her in such words before. She had
always thought of herself as a dull, uninteresting woman. Now, suddenly, to be told such things, and by
such a handsome man at that. To become a
Lady, and mix with the social elite of the kingdom. Her head was spinning. What did it signify? Did he truly mean what he had said, or,
perhaps, was he simply trying to seduce her with sweet words? Catherine had heard of such things.
“Why, yes,” Lord Colon continued, “you are
the prettiest lady in all of the heath, and would make me the finest wife, from
the river Eyre to the tall mountains in the north.”
“Why, sir, you flatter me, but to what
purpose?” Catherine was beginning to
enjoy exchanging words with the handsome Captain of the Horse Guards.
“My purpose you ask, my lady? Only to win your heart!” Declared the handsome young man. He swept his hat from his head and bowed low
from the waist. “I must win your heart
to replace the very heart which I have lost to you.”
“Lost your heart? Why what can you mean?” Catheri
Drat: Well, it’s happened
again. The Physics Kahuna is perfectly
aware that the preceding paragraphs are utter nonsense and have no place at all
in a quality work of science education such as the various chapters you have
been studying throughout the year. A
most sincere apology is offered.
We have done a lot of work with energy, but
the type of energy we’ve dealt with has been mechanical energy. It is time now to look into other types of
energy. Thermal energy has to do with
the internal energy of a system – the energy of the particles that make a thing
up.
Thermal
Energy º The total kinetic energy of the particles in a system
The particles that make up a system are the
molecules, atoms, or ions that make it up.
These particles have kinetic energy – they have motion. In a gas, the particles are free to zoom
around and bounce off other particles.
In a liquid they also flit about, but they also make weak bonds with
each other and tend to clump together.
In a solid the particles are bound together with chemical bonds. These bonds are not rigid, however, so the
particles can move back and forth. Kind
of like they’re connected to each other with springs that allow them to
vibrate.
Thermal
Definitions:
Here are some important definitions:
Thermal
Contact º two systems are placed so
that they can exchange thermal Energy
Heat º thermal energy transferred
from one system to another.
Temperature º average kinetic energy of
the particles in a system.
Thermal
equilibrium º A static state. Objects in thermal contact reach the same
average internal energy state and no longer exchange thermal energy.
Temperature: Instruments that measure
temperature are called thermometers.
There are several temperature scales that are in use around the world -
the Celsius scale, Fahrenheit scale, Kelvin scale, and the Rankine scale are
the major ones.
We will make use of the Celsius scale and
the Kelvin scale. The other two scales
are only used in the US of A, so we can safely ignore them. (Who cares about a country so backward that
they don’t even use the metric system?)
The Celsius scale was originally set up to
monitor temperatures on the earth’s surface.
The zero point on the scale is fixed at the freezing temperature of
water and 100° C is the boiling temperature of the
good old H2O (at one atmosphere pressure). The zero on this scale is arbitrary and has
no physical meaning as a zero value (zero is supposed to be when you have
“nothing”, right?)
Temperatures using the Celsius scale are
reported as degrees Celsius. One
would say, “Hey, the temperature today is twenty-three degrees Celsius!
The Kelvin scale has a true zero, its zero
value represents the lowest possible temperature, which is known as absolute
zero. Absolute zero represents
the minimum possible energy state for matter.
When reporting a Kelvin temperature, one
would say, “Hey it’s three hundred and one Kelvins outside! You don’t use the word “degree” with a Kelvin
temperature.
The size of the units for each scale are
the same.
Absolute zero, which is zero Kelvins,
is –273.15°C. The freezing point of
water, 0°C is 273.15 K. Converting between the scales is simple, to
convert Celsius to Kelvins you just add 273.15 and to convert Kelvins to
Celsius you subtract 273.15.
When an object absorbs heat, its particles
must somehow gain kinetic energy. They
do this by absorbing heat. There are
three ways that heat can be transferred between systems: conduction, radiation, and convection. The Physics Kahuna hopes that you are
familiar with these. This is the kind of
stuff that you do in elementary science classes.
Conduction is heat
transfer by direct contact between two systems.
When you sit on a really hot car seat and burn your hide, you have
gained heat via conduction. Heat will
flow from the hot system to the cold system until thermal equilibrium is
reached. At that point heat will no
longer flow.
What happens in conduction is that the
particles in the hot object, placed against the cold object, have collisions
with the particles in the cooler system.
In these collisions, the particles in the high temperature system lose
energy and the particles in the low temperature system gain energy. The thermal energy transfers through the cool
system via collisions between the particles until the particles in the two
systems have the same average kinetic energy.
We say that the systems have reached thermal equilibrium when this
happens.
Radiation is heat
transfer via electromagnetic waves. A
great deal of the energy from the sun reaches the earth in the form of
electromagnetic waves. They travel
through space. When you go outside on a
warm spring day and bask in the warmth of the sun, you are absorbing heat that
has radiated from the sun. Electric
space heaters that have those glowing red heat elements provide most of their
heat via radiation in the same way. Most
of the heat that you get from a fireplace (or a camp fire for that matter)
arrives via radiation.
The electromagnetic waves travel through
space and are absorbed by the system, the absorbed energy is converted into the
kinetic energy of the particles – makes them move back and forth.
Convection is heat
transfer via fluid flow. A fluid absorbs
heat at one location and then flows to another place where it transfers the
heat it absorbed to some other system.
Convection is used to heat homes via forced air furnaces. A large fan blows warm air into the rooms of
the house through ducts. Cooler air is
drawn in through grates and returned to the furnace for heating.
Ovens do most of their heating via
conduction. An element in the bottom of
the oven heats air. The heated air then
circulates around and around in the oven transferring heat to the food you want
to cook.
It is quite common to have multiple forms
of heat transfer in a system. Burning
gasoline in a car engine heats the engine block by conduction. Water is circulated through water channels in
the engine block absorbing heat via conduction.
It then carries the heat to the radiator – this is convection. The heat is then transferred from the water
to the radiator via conduction. Air
circulates through the radiator removing heat via conduction (when the air is
in direct contact with the metal fins of the radiator). The heated air then departs, removing the
heat from the radiator via convection.
Heat
Transfer by Conduction: Materials that allow
heat to flow through them easily are called heat conductors,
materials which do not allow heat to flow through them are called heat
insulators.
Heat conductors are things like
metals. Metals are good conductors
because of the nature of the chemical bond that binds the atoms together. These bonds are called metallic bonds. The significant thing about the bonds are
that some of the electrons of each atom are not bound to any one particular
atom – they’re kind of like “community” electrons belonging to everyone. They are very loosely held and can move
around throughout the metal. These are
known as free electrons. The free
electrons carry the heat from one part of the metal to another. They do this via collisions wherein one
electron gives some of its energy to another.
This can happen very quickly so that the heat transfers quite easily.
Insulators do not have handy little
particles that can collide with each other transferring energy from one place
to another. There are no free electrons
to do this. If it is a solid, then the
electrons are going to be tightly held via covalent or ionic bonds.
Gases make very good insulators because
there aren’t many particles to carry the energy from one place to another.
Clearly a vacuum would be even better!
Most insulators are actually materials that
have lots of little pockets of air (or a fancy gas) in them. The gas slows the flow of heat way down.
Thermos bottles are very interesting. You can put a hot fluid in the thing and it
will stay hot or you can put a cold fluid in it and the fluid will stay
cold. The question is this, “How does
the bottle know what to do?” Maybe a
microchip thingee? Hmmm. Well, actually, thermos bottles are very
simple devices. The have an external
metal or plastic body, inside of this body is a glass bottle. Really good thermos bottles have a vacuum
between the outer case and glass bottle.
The glass bottle is mirrored.
Heat is kept from flowing by the vacuum which prevents conduction and
convection. The mirror surface prevents
heat flow by radiation – the mirror reflects the electromagnetic waves
(infrared waves, right?). So thermos
bottles do a pretty good job of blocking the flow of heat coming either into or
out of the bottle. So it can keep hot
things hot and cold things cold – without microprocessors.

One of the units used to measure heat was
the calorie. A calorie is the amount of
heat it takes to raise the temperature of one cubic centimeter of water by one
degree Celsius. Joule found that heat
could be related to mechanical energy.
Here is a description of Joule’s elegant experiment. An insulated container was filled with water. The temperature of the water could be monitored with a thermometer. In the water tank was a set of paddles that could rotate. A piece of line was attached to the paddles and run over a pulley system. A weight was attached to the line and released. The weight would fall down, causing the paddle to rotate in the water, thus doing work. The temperature of the water increased, indicating that heat had entered the system, even though it was insulated. Joule found that the amount of work done by the weight was equal to the thermal energy that increased the water’s temperature.
The relationship between the calorie and the joule is:
The relationship between the calorie and the joule is:
Heat
and Temperature Change: The heat required to raise the temperature of
a substance can be looked at in several ways.
The way we look at it in physics is to use a thing called the specific
heat.
Specific Heat º heat to raise the temperature of one gram of a substance by one degree Celsius.
The specific heat can be found experimentally, but usually you just look it up in a table. This elegant document has just such a table – the very thing! The Physics Kahuna thinks that it is on the next page or else one of the others.
The heat required to raise a substance’s temperature is given by this equation:
Specific Heat º heat to raise the temperature of one gram of a substance by one degree Celsius.
The specific heat can be found experimentally, but usually you just look it up in a table. This elegant document has just such a table – the very thing! The Physics Kahuna thinks that it is on the next page or else one of the others.
The heat required to raise a substance’s temperature is given by this equation:
Exothermic phase changes give off heat. Freezing and condensation are exothermic.
When a substance is changing state, its temperature remains constant. Heat is being added or is leaving, but the heat doesn’t affect the temperature, instead it is involved in either breaking or forming bonds.
Heat added to cause a phase change is called latent heat (latent meaning hidden).
The heat needed to cause vaporization is called the latent heat of vaporization. Usually this is shortened to just “heat of vaporization”. It works for both vaporization and condensation. When the substance condenses after being vaporized, it gives off this heat.
The heat involved in melting or freezing is called the latent heat of fusion. Shortened to “heat of fusion”.
The heat required for a phase change of a substance is given by this equation:
Q is heat, m is mass, and L is the latent heat for the phase
change.
The latent heat of vaporization has this symbol: Lv
The latent heat of fusion has this symbol: Lf
Here’s a handy table of values:
The latent heat of vaporization has this symbol: Lv
The latent heat of fusion has this symbol: Lf
Here’s a handy table of values:
|
Substance
|
Melting Point
°C
|
Heat of Fusion
kJ/kg
|
Boiling Point
°C
|
Heat of Vaporization
kJ/kg
|
|
Helium
|
- 269.65
|
5.23
|
-268.93
|
20.9
|
|
Nitrogen
|
- 209.97
|
25.5
|
-195.81
|
201
|
|
Oxygen
|
-218.79
|
13.8
|
182.97
|
213
|
|
Ethyl Alcohol
|
-114
|
104
|
78
|
854
|
|
Ammonia
|
-75
|
452
|
2 870
|
1 370
|
|
Water
|
0.00
|
333
|
100.00
|
2
256
|
|
Sulfur
|
119
|
38.1
|
444.6
|
326
|
|
Lead
|
327.3
|
24.5
|
1750
|
870
|
|
Aluminum
|
660
|
397
|
2450
|
11 400
|
|
Silver
|
960.80
|
88.2
|
2193
|
2 330
|
|
Gold
|
1063.0
|
64.4
|
2660
|
1
580
|
|
Copper
|
1083.0
|
134
|
1187
|
473
|
|
Mercury
|
-38.87
|
11.8
|
356.58
|
296
|
|
Tin
|
232
|
60.3
|
2270
|
2 200
|
|
Tungsten
|
3410
|
180
|
5927
|
824 kj/mole
|
|
Iron
|
1535
|
33.0
|
3000.0
|
6 700
|
|
Zinc
|
419.4
|
96.3
|
907
|
199
|
Heat/Temperature
Curves: When
a graph is made up temperature vs heat, the curve will look like the generic one
below.

The slopes represent the specific heat of
the different phases.
The flat parts of the graph where the
temperature does not increase with added heat represent the phase changes. The
heat added is latent heat.
Where the graph has a slope, the temperature
does increase with energy, so the heat added is sensible heat.
One can find the value of the boiling and
melting temperatures by finding the flat areas where the phase changes.
·
How much
heat is required to melt 2.85 kg of ice at zero degrees Celsius?
This is a simple problem. Just use the phase change equation.
Dear Doctor
Science,
If I were to open my freezer door, and then the door to my hot 450 degree oven simultaneously, would not the warm and cold fronts converge in my kitchen, creating miniature tornadoes on the linoleum floor?
-- David from River Hills, WI
Dr. Science responds:
Indeed, this is how most weather forecasters amuse themselves, when they're not playing "guess the barometric pressure" or "pin the tail on the correct cloud formation." It's best to sweep the kitchen floor before you unloose hundreds of miniature tornadoes, because dust and crumbs accelerated to hundreds of miles an hour can punch a hole in your cabinets. It's a good way to terrorize roaches or ants that have previously walked with impunity on your kitchen floor. Suddenly they're playing Wizard of Oz and chirping "there's no place like home."
Dear Dr. Science,
Many people realize they can tell the temperature by counting the chirps a cricket makes. But how does the cricket know what temperature it is?
------ Brian W., Laramie, Wyoming
Dr. Science responds:
Brian, while you're out on the veranda swatting mosquitoes and complaining to your friends about how hot it is, the cricket sits in air-conditioned comfort watching the evening news. Out of boredom, perhaps, or a genuine need to give us information, the cricket communicates this weather data to you. The cricket will also click out (in Morse code) the final sports scores, national headlines and such phrases as "Now this", "Coming up at 11," or "Our White House Correspondent filed this report." Some scientists call the cricket the Ted Koppel of the insect world, which is accurate but somewhat silly. After all, you'll never see Ted Koppel rubbing his legs together. At least I hope you won't.
If I were to open my freezer door, and then the door to my hot 450 degree oven simultaneously, would not the warm and cold fronts converge in my kitchen, creating miniature tornadoes on the linoleum floor?
-- David from River Hills, WI
Dr. Science responds:
Indeed, this is how most weather forecasters amuse themselves, when they're not playing "guess the barometric pressure" or "pin the tail on the correct cloud formation." It's best to sweep the kitchen floor before you unloose hundreds of miniature tornadoes, because dust and crumbs accelerated to hundreds of miles an hour can punch a hole in your cabinets. It's a good way to terrorize roaches or ants that have previously walked with impunity on your kitchen floor. Suddenly they're playing Wizard of Oz and chirping "there's no place like home."
Dear Dr. Science,
Many people realize they can tell the temperature by counting the chirps a cricket makes. But how does the cricket know what temperature it is?
------ Brian W., Laramie, Wyoming
Dr. Science responds:
Brian, while you're out on the veranda swatting mosquitoes and complaining to your friends about how hot it is, the cricket sits in air-conditioned comfort watching the evening news. Out of boredom, perhaps, or a genuine need to give us information, the cricket communicates this weather data to you. The cricket will also click out (in Morse code) the final sports scores, national headlines and such phrases as "Now this", "Coming up at 11," or "Our White House Correspondent filed this report." Some scientists call the cricket the Ted Koppel of the insect world, which is accurate but somewhat silly. After all, you'll never see Ted Koppel rubbing his legs together. At least I hope you won't.
At home we play this little game of placing an ice cube on a smooth table and then shaking some salt on top of it. After a about 30 seconds the ice is stuck to the table. It sometimes requires a lot of force to dislodge it. Why does this happen?
--- john lutz from Seattle, WA
Dr. Science responds:
I share your queer idea of fun. My lab assistant and I used to sprinkle salt on slugs, until someone reported us for mollusk abuse, and we were forced to take sensitivity training so mind numbing it almost cost me my sanity. In your little game, the ice sticks to the table because salt is a natural aphrodisiac, causing the ice to mate with the table. Yes, salty water is randy water, which is why the sea is so often used as a symbol of romance. People sigh when they look out at the sea, and
often feel a lump in their, uh, throat and an ache in their heart, both of which are often signs of ozone poisoning, caused by temperature inversions trapping smog along the shoreline, but you probably knew that already.
Dear Doctor Science,
The defroster in my car doesn't work very well and I'm often forced to scrape the frost off the inside of my windshield while I'm driving. Why is there always more frost directly in front of me than in any other area of the windshield?
-- Brian Price from Norfolk, VA
Dr. Science responds:
Your car is trying to kill you. If I were you, I'd trade it in as soon as possible. This defroster malfunction is only the tip of the iceberg, so to speak. One day the brake pedal will be suspiciously soft, and then when you're heading into a curve you'll find you have no brakes at all. I once heard of a Saab that fried its owner with the driver's seat warmer. By the way, never stick your head through a sun roof, even in jest. Those things can close very quickly, even with no one at the control. Yes, new cars are intelligent, flashy, and unbelievably malevolent.
Dear Cecil:
I am a member of a small group which meets Sunday afternoons to read aloud the novels of Charles Dickens. Last week we reached chapter 32 of Bleak House. In this chapter, a rather low character by the name of Krook dies by--get this--spontaneous combustion. All that remains of him is a small heap of cinder and ash.
I was delighted, but since then I have been looked at rather pathetically by everyone I've reported it to. No one believes it to be possible. Well, Cecil, if it is an actual phenomenon, then why hasn't anyone heard of it? If it isn't, how did the notion start and how was our dear Mr. Dickens led astray? --Scott E., Chicago
Cecil replies:
Spontaneous human combustion (SHC for short) is one of those twilight zone-type phenomena that people tend to lump with ectoplasm and telekinesis, so discussion has been confined largely to the nutcake journals. Nonetheless, a considerable body of evidence suggests that something like SHC actually occurs.
Over the past 300 years, there have been more than 200 reports of persons burning to a crisp for no apparent reason. The victims are discovered as piles of ashes and oily residue, completely consumed except for an occasional unburnt arm or leg.
Although temperatures of about 3,000 degrees Fahrenheit are normally required to char a body so thoroughly (crematoria, which usually operate in the neighborhood of 2,000 degrees, leave bone fragments which must be ground up by hand), frequently little or nothing around the victim is damaged, except perhaps the exact spot where the deceased ignited. SHC victims have burnt up in bed without the sheets catching fire, clothing worn is often barely singed, and flammable materials only inches away remain untouched.
According to researcher Larry Arnold, the first medical report of SHC appeared in Acta Medica & Philosophica Hafniensia in 1673. A hard-drinking Parisian was found reduced to ashes in his straw bed, leaving just his skull and finger bones. The straw matting was only lightly damaged. Since then many other occurrences have been noted. Charles Dickens, in doing research for Bleak House, found 30 cases on record.
Here are some typical SHC reports:
I am a member of a small group which meets Sunday afternoons to read aloud the novels of Charles Dickens. Last week we reached chapter 32 of Bleak House. In this chapter, a rather low character by the name of Krook dies by--get this--spontaneous combustion. All that remains of him is a small heap of cinder and ash.
I was delighted, but since then I have been looked at rather pathetically by everyone I've reported it to. No one believes it to be possible. Well, Cecil, if it is an actual phenomenon, then why hasn't anyone heard of it? If it isn't, how did the notion start and how was our dear Mr. Dickens led astray? --Scott E., Chicago
Cecil replies:
Spontaneous human combustion (SHC for short) is one of those twilight zone-type phenomena that people tend to lump with ectoplasm and telekinesis, so discussion has been confined largely to the nutcake journals. Nonetheless, a considerable body of evidence suggests that something like SHC actually occurs.
Over the past 300 years, there have been more than 200 reports of persons burning to a crisp for no apparent reason. The victims are discovered as piles of ashes and oily residue, completely consumed except for an occasional unburnt arm or leg.
Although temperatures of about 3,000 degrees Fahrenheit are normally required to char a body so thoroughly (crematoria, which usually operate in the neighborhood of 2,000 degrees, leave bone fragments which must be ground up by hand), frequently little or nothing around the victim is damaged, except perhaps the exact spot where the deceased ignited. SHC victims have burnt up in bed without the sheets catching fire, clothing worn is often barely singed, and flammable materials only inches away remain untouched.
According to researcher Larry Arnold, the first medical report of SHC appeared in Acta Medica & Philosophica Hafniensia in 1673. A hard-drinking Parisian was found reduced to ashes in his straw bed, leaving just his skull and finger bones. The straw matting was only lightly damaged. Since then many other occurrences have been noted. Charles Dickens, in doing research for Bleak House, found 30 cases on record.
Here are some typical SHC reports:
- On April 9, 1744, Grace Pett, 60, an alcoholic residing in Ipswich, England, was found on the floor by her daughter like "a log of wood consumed by a fire, without apparent flame." Nearby clothing was undamaged.
- On May 18, 1957, Anna Martin, 68, of West Philadelphia, Pennsylvania, was found incinerated, leaving only her shoes and a portion of her torso. The medical examiner estimated that temperatures must have reached 1,700 to 2,000 degrees, yet newspapers two feet away were found intact.
- On December 5, 1966, the ashes of Dr. J. Irving Bentley, 92, of Coudersport, Pennsylvania, were discovered by a meter reader. Dr. Bentley's body apparently ignited while he was in the bathroom and burned a 2-1/2-by-3-foot hole through the flooring, with only a portion of one leg remaining intact. Nearby paint was unscorched.
The police report declared that Mrs. Reeser went up in smoke when her highly flammable rayon-
acetate nightgown caught fire, perhaps
because of a dropped cigarette. But one medical observer declared that the
3,000-degree heat required to destroy the body should have destroyed the
apartment as well. In fact, damage was minimal--the ceiling and upper walls
were covered with soot. No chemical accelerants, incidentally, were found.
No satisfactory explanation of SHC has been offered. Many SHC victims have been alcoholics, and at one time it was thought that alcohol or its derivatives in the body simply ignited. But experiments in the 19th century demonstrated that flesh impregnated with alcohol will not burn with the intense heat associated with SHC. Other theories involve deposits of flammable body fat--many victims have been overweight. But others have been skinny.
One school of thought blames phosphorous. One of the Teeming Millions explains: "SHC is thought to be the result of an error in phosphorous metabolism. As you may recall from your college biochemistry, living creatures store accessible energy in phospho-diester bonds. Under certain conditions, improperly manufactured polyphosphorous compounds in all the body cells can undergo an autocatalytic reaction. Water will not stop SHC. To get an idea of what's happening, have a chemist drop polyphosphoric acid in water." Unfortunately, I have scoured the recent scientific literature in vain for any discussion along these lines. Biochemists I have spoken to reject the idea out of hand.
So the question remains open. At least nobody's claiming that UFOs or the spirit world are involved. You may rely on Uncle Cecil to keep you abreast of future developments.
UPDATE
I said I'd keep you up to date on spontaneous human combustion (SHC). You thought I was kidding?
Past SHC researchers have blamed everything from excessive alcohol consumption to "geomagnetic fluctuations." Now Joe Nickell and John Fischer, the former a well-known investigator of the paranormal, have analyzed the evidence in 30 cases and concluded that SHC may not be so inexplicable after all.
Here's a rundown of their findings, as published in the Skeptical Inquirer:
No satisfactory explanation of SHC has been offered. Many SHC victims have been alcoholics, and at one time it was thought that alcohol or its derivatives in the body simply ignited. But experiments in the 19th century demonstrated that flesh impregnated with alcohol will not burn with the intense heat associated with SHC. Other theories involve deposits of flammable body fat--many victims have been overweight. But others have been skinny.
One school of thought blames phosphorous. One of the Teeming Millions explains: "SHC is thought to be the result of an error in phosphorous metabolism. As you may recall from your college biochemistry, living creatures store accessible energy in phospho-diester bonds. Under certain conditions, improperly manufactured polyphosphorous compounds in all the body cells can undergo an autocatalytic reaction. Water will not stop SHC. To get an idea of what's happening, have a chemist drop polyphosphoric acid in water." Unfortunately, I have scoured the recent scientific literature in vain for any discussion along these lines. Biochemists I have spoken to reject the idea out of hand.
So the question remains open. At least nobody's claiming that UFOs or the spirit world are involved. You may rely on Uncle Cecil to keep you abreast of future developments.
UPDATE
I said I'd keep you up to date on spontaneous human combustion (SHC). You thought I was kidding?
Past SHC researchers have blamed everything from excessive alcohol consumption to "geomagnetic fluctuations." Now Joe Nickell and John Fischer, the former a well-known investigator of the paranormal, have analyzed the evidence in 30 cases and concluded that SHC may not be so inexplicable after all.
Here's a rundown of their findings, as published in the Skeptical Inquirer:
- In most cases combustion probably wasn't spontaneous. Candlesticks, oil lamps, pipes, and the like were often found near the victims. Mrs. Reeser when last seen alive was smoking a cigarette.
- The victims tended to be slow to react. Many were alcoholics; others were elderly, overweight, or handicapped in some way. Mrs. Reeser was 67, weighed 175 pounds, and had a bad leg. The evening before her demise she told her son she had taken two sleeping pills and expected to take two more.
- Bodies can be totally consumed at temperatures much lower than previously believed. Proponents of paranormal explanations for SHC often point out that crematoriums use temperatures of 2,000 degrees or more, much hotter than the usual household fire. But experts say high temps are necessary only if the body must be destroyed in a short time. Smoldering fires can consume an entire piece of furniture (and presumably the body within it) if given long enough. Yet they often leave nearby objects undamaged. Twelve hours passed between the time Mrs. Reeser was last seen alive and the time her remains were discovered.
- In cases where the body was completely destroyed, there was often a nearby source of combustible material to feed the fire. The floorboards beneath a number of victims were found burnt through; Mrs. Reeser was wearing a flammable nightgown and housecoat and was sitting in an overstuffed chair. In addition--this gets pretty gross--the fuel sources may have served to catch melting body fat which then added to the flames. Call it the "candle effect." A quantity of "grease," Nickell and Fischer note, was found where Mrs. Reeser's chair had stood.
"In the Reeser case, what probably
happened was that the chair's stuffing burned slowly, fueled by the melted body
fat and aided by partially open windows," Nickell and Fischer conclude.
"What has been described as 'probably the best-documented case' of alleged
spontaneous human combustion is actually attributable to the deadly combination
of a lit cigarette, flammable nightclothes, and sleeping pills."
Grisly stuff, but I thought you'd want to know.
--CECIL ADAMS
Grisly stuff, but I thought you'd want to know.
--CECIL ADAMS
Invictus
Out of the night that covers me,
Black as the Pit from pole to pole,
I thank whatever gods may be
For my unconquerable soul.
In the fell clutch of circumstance
I have not winced nor cried aloud.
Under the bludgeonings of chance
My head is bloody, but unbowed.
Beyond this place of wrath and tears
Looms but the horror of the shade,
And yet the menace of the years
Finds, and shall find me, unafraid.
It matters not how strait the gate,
How charged with punishments the scroll,
I am the master of my fate;
I am the captain of my soul.
---- Williams Ernest Henley
Requiem
Under the wide and starry sky
Dig the grave and let me lie:
Glad did I live and gladly die,
And I laid me down with a will.
This be the verse you grave for me:
Here he lies where he long’d to be;
Home is the sailor, home from the sea,
And the hunter home from the hill.
-- Robert Louis Stevenson
Not in Vain
If I can stop one heart from breaking,
I shall not live in vain:
If I can ease one life the aching,
Or cool one pain,
Or help one fainting robin
Unto his nest again,
I shall not live in vain.
-- Emily Dickinson






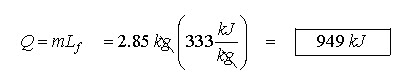
0 comments:
Post a Comment